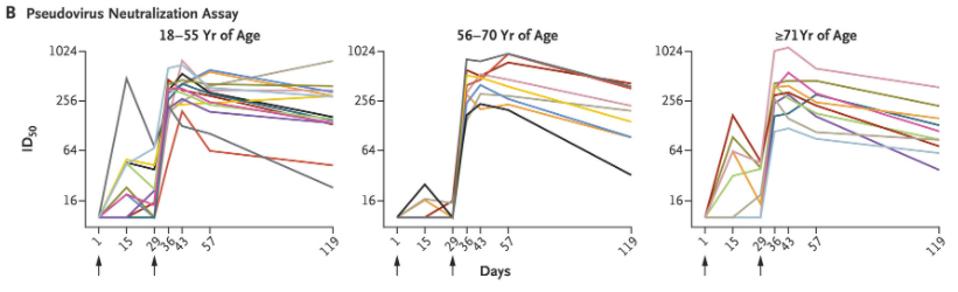
Pennsylvania Enters 1B Phase Of COVID-19 Vaccination Timeline First responders postal workers people in correctional facilities people in homeless shelters manufacturing workers and public transit workers are among those now eligible. But its not clear yet when those workers - who are in Phase 1B - will be vaccinated.
 This Map Shows Where To Find Covid 19 Vaccine Providers In Pa Witf
This Map Shows Where To Find Covid 19 Vaccine Providers In Pa Witf
First responders critical workers and people with high-risk conditions will be vaccinated.
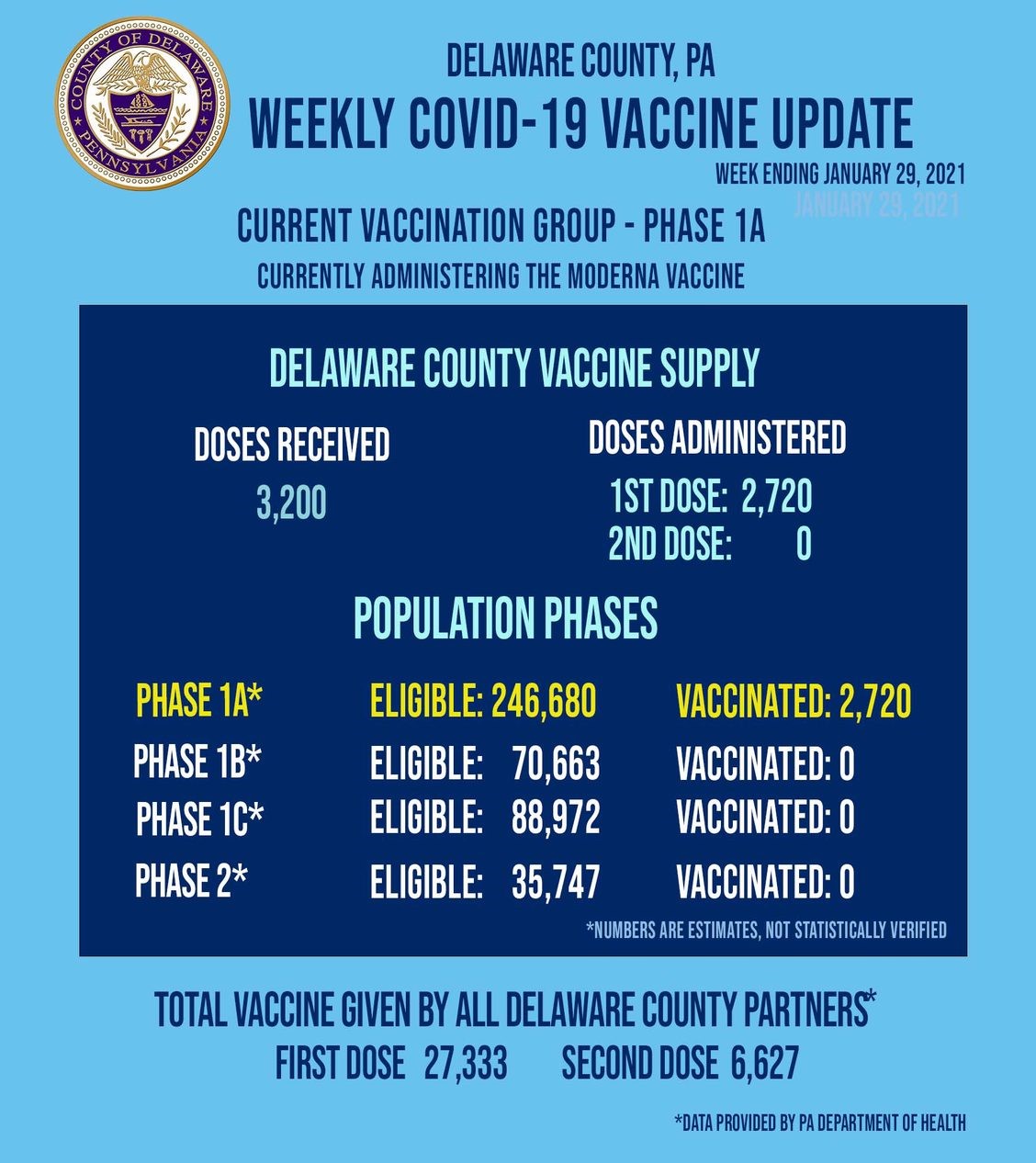
Pa vaccine phase 1b. Demand for the vaccine still exceeds the supply Beam said. The Department will utilize enrolled partnerships with. Ryan Aument R-Lancaster County a member of Pennsylvanias vaccine task force said more workers in Phase 1B could be next in line to get the one-dose Johnson.
The timeline will be determined by how quickly everyone who is. More Pennsylvania residents can now receive the COVID-19 vaccine as the state moves to Phase 1B of its vaccine rollout MondayIncluded in Phase 1B. The COVID-19 vaccine can keep you from getting COVID-19 and is supposed to lessen symptoms if you do contract the virus.
Pennsylvania moves into Phase 1B of vaccine rollout. Once the state finishes its educator vaccination campaign with Johnson Johnson shots the single-dose vaccines will go to certain Phase 1B workers like law enforcement firefighters grocery. After spending roughly four months in the first phase of its COVID-19 vaccine rollout Pennsylvania will move to phase 1B on Monday which expands eligibility to.
PITTSBURGH The Pennsylvania Department of Health has updated its plan to distribute the coronavirus vaccine in phases. COVID-19 vaccination will help protect you by creating an antibody response without having to experience sickness. April 5 2021 915 AM.
Pennsylvania COVID-19 vaccines will be open to everyone by April 19 with Phase 1B beginning April 5 and Phase 1C on April 12. Wolf announces push to finish phase 1A of COVID-19 vaccine rollout and plan to vaccinate police grocery workers other groups. In response to questions from PennLive Wolf administration Sara Goulet said.
But not until April 19 will the state move into Phase 2 which will open up vaccines. Phase 1C which begins April 12 includes financial workers and informational technology and communications workers. People who fall into the states Phase 1B category can now get vaccinated against COVID-19 as of.
And anyway she said plenty of people in 1B like teachers and daycare workers and soon supermarket workers law. The Pennsylvania Department of Health announced the. Phase 1B includes food agricultural grocery store and education workers.
More Pennsylvanians are now eligible for the COVID-19 vaccine. Matthews Tavares lead Maple Leafs to 4-2 win over Flames. Right now Pennsylvania is in Phase 1B but as of.
Pennsylvanians in Phase 1B Now Eligible for COVID-19 Vaccine April 05 2021 Press Release Public Health Public Safety Starting today Pennsylvanians in Phase 1B of the states vaccination plan are now eligible to schedule a COVID-19 vaccination appointment to become protected against the virus. State health officials said its still too early to put a date on when Pennsylvania will move to Phase 1B of the vaccine rollout. - Vaccine eligibility in Pennsylvania is expanding to more adults.
With more than 17 million people fully vaccinated in PA including thousands of teachers school staff some are asking why havent we moved into Phase 1B. WHITEHALL TWP Pa. Vaccines are safe and are the best way to protect yourself and those around you from serious illnesses.
By Lindsay Ward April 5 2021 at 511 am. But if the governor is correct and the teacher vaccination effort will be done by the end of next week vaccinations for those in Phase 1B could begin in mid-April.