Rapid tests such as Abbotts are generally less accurate than molecular diagnostic tests which are the industry gold standard but must be sent to a laboratory to produce results a process that. On its website and in news releases Abbott maintains its test performs best in patients tested earlier post symptom onset.
 Abbott S Fast 5 15 Minute Easy To Use Covid 19 Antigen Test Receives Fda Emergency Use Authorization Mobile App Displays Test Results To Help Our Return To Daily Life Ramping Production To 50 Million Tests A
Abbott S Fast 5 15 Minute Easy To Use Covid 19 Antigen Test Receives Fda Emergency Use Authorization Mobile App Displays Test Results To Help Our Return To Daily Life Ramping Production To 50 Million Tests A
Agilent is a leader in the life sciences diagnostics and applied chemical markets.

Abbott rapid test accuracy. Instead of taking hours to deliver a result the Abbott testswhich detect viral proteinscould provide an answer within 15 minutes. Food and Drug Administration issued an alert Thursday about the accuracy of Abbott Labs rapid Covid-19 test after researchers at New York. Last week researchers at New York Universitys health system published a report showing accuracy problems with Abbotts rapid coronavirus test.
These tests often come as flat plastic card that contain a test strip. How accurate is it. Ad Agilent delivers better scientific and business outcomes for labs.
Previously antigen tests were thought to. Several independent research teams have called into question the accuracy of Abbotts ID NOW COVID-19 test a rapid point-of-care molecular diagnostic test designed to produce results in five minutes. Rapid antigen tests have received Food and Drug Administration FDA Emergency Use Authorization EUA for use in symptomatic persons 2 but data are lacking.
The test strip reacts with coronavirus antigens present in the patients mucus sample and the strip typically changes color to indicate that the patient is positive. The Abbott rapid test can still be used to identify positive COVID-19 cases according to Stenzel though negative results may need to go through a secondary process to. To a certain degree youre sacrificing accuracy with speed.
FDA warns on accuracy of Abbott rapid COVID-19 test May 15 2020 By Nancy Crotti Abbotts ID Now test machine Image from Abbott After weeks. According to Abbott BinaxNOW has a correct positive diagnosis rate of 971 and a correct negative diagnosis rate of 985 when. Rapid antigen tests such as the Abbott BinaxNOW COVID-19 Ag Card BinaxNOW offer results more rapidly approximately 1530 minutes and at a lower cost than do highly sensitive nucleic acid amplification tests NAATs 1.
Health Minister Patty Hajdu who previously questioned the accuracy of antigen tests said the new Abbott device will take pressure off overrun testing sites and offer specific advantages for. Abbotts ID NOW COVID-19 test is promoted as delivering positive test results in five minutes and negative results in about 13 minutes. Agilent is a leader in the life sciences diagnostics and applied chemical markets.
Are rapid COVID-19 tests reliable. Shortly after Abbott completed its delivery of 150 million rapid antigen tests to the federal government for widespread distribution against the COVID-19 pandemic researchers at the Centers for. The government planned to send them to.
Ad Agilent delivers better scientific and business outcomes for labs. It is an automated assay that utilizes isothermal nucleic acid amplification technology. ID Now COVID-19 Abbott Diagnostics Scarborough Inc Scarborough ME is a rapid test that qualitatively detects SARS-CoV-2 viral nucleic acids from nasal nasopharyngeal and throat swabs.
The Abbott rapid antigen test authorized in August reports a 971 percent sensitivity rate which is very promising. FDA granted Abbott an emergency use authorization of the test in late March. Tests were most accurate when used in the first week after symptoms first developed an average of 78 of confirmed cases had positive antigen tests.
This is likely to be because people have the most virus in their system in the first days after they are infected.
 Diagnostic Accuracy Of The Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared With Reverse Transcriptase Polymerase Chain Reaction Testing Of Nasopharyngeal Samples In The Pediatric Population The Journal Of
Diagnostic Accuracy Of The Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared With Reverse Transcriptase Polymerase Chain Reaction Testing Of Nasopharyngeal Samples In The Pediatric Population The Journal Of
 As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
 Florida Eyes Abbott Rapid Test As Game Changer Miami Herald
Florida Eyes Abbott Rapid Test As Game Changer Miami Herald
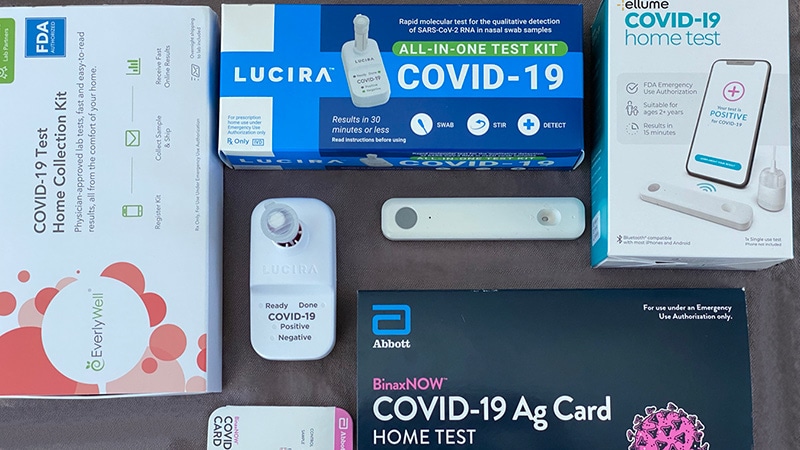 Rapid Covid Tests Are Coming To Stores Near You
Rapid Covid Tests Are Coming To Stores Near You
 Abbott Launches Rapid Portable Antigen Test Increasing Covid 19 Testing Capacity In France 2020 09 18 Bioworld
Abbott Launches Rapid Portable Antigen Test Increasing Covid 19 Testing Capacity In France 2020 09 18 Bioworld
 Fda Cautions About Accuracy Of Widely Used Abbott Coronavirus Test Coronavirus Updates Npr
Fda Cautions About Accuracy Of Widely Used Abbott Coronavirus Test Coronavirus Updates Npr
 Abbott Panbio Covid 19 Rapid Test Corona Antigen Test Nose Swab Medische Vakhandel
Abbott Panbio Covid 19 Rapid Test Corona Antigen Test Nose Swab Medische Vakhandel
 Covid 19 Rapid Test By Abbott Labs Given Emergency Fda Approval Claims Accurate Results In 15 Minutes Abc7 Chicago
Covid 19 Rapid Test By Abbott Labs Given Emergency Fda Approval Claims Accurate Results In 15 Minutes Abc7 Chicago
 Panbio Covid 19 Ag Rapid Test Device Abbott Point Of Care Testing
Panbio Covid 19 Ag Rapid Test Device Abbott Point Of Care Testing
 Fda Setujui Rapid Test Covid 19 Seharga Rp350 Ribu Buatan Abbot Bisa Dipakai Di Rumah Lifestyle Bisnis Com
Fda Setujui Rapid Test Covid 19 Seharga Rp350 Ribu Buatan Abbot Bisa Dipakai Di Rumah Lifestyle Bisnis Com
 Rapid Covid Tests More Important Than Ever Abbott Newsroom
Rapid Covid Tests More Important Than Ever Abbott Newsroom
 Fda Issues Alert On Accuracy Of Abbott Id Now Rapid Test
Fda Issues Alert On Accuracy Of Abbott Id Now Rapid Test
 Abbott S 5 Rapid Covid 19 Antigen Test Granted Authorization By Fda
Abbott S 5 Rapid Covid 19 Antigen Test Granted Authorization By Fda
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.